Milkdrop Clinician Program
Welcome! We’re thrilled you’re interested in joining the Milkdrop Clinician Program.
This page provides more information for Lactation Consultants, Midwives, Doctors and Physical Therapists on Milkdrop cushions, including clinical observations, medical device registration and usage.
About the program
Join a growing community of clinicians who are finding Milkdrop helpful for making pumping more comfortable and sustainable for their clients.
When you join the program you will receive:
- A sample Milkdrop cushion in your choice of size (XS, S, M) for you to check out
- Educational materials on how to use the cushion
- A unique discount code for your clients
- Potential giveaway opportunities to expand your audience reach
FAQs
Who is Milkdrop?
We are a small team of Australian engineers and designers, led by Alex, who pumped to feed her daughter. We are working on making pumping more comfortable, and trying to innovate around how flanges fit and adapt to the breast. We'd love to hear from you, and learn about what you see (and think might help) in your practise!
What is the Milkdrop cushion?
The cushion is a soft silicone cushion that stretches over the head of most traditional breast pumps (and soon for new 'wearable' pumps too).
It is designed to provide a softer interface between the breast and the existing breast pump.
Why did you design it?
Whilst feeding at the breast is typically clinically preferable to using breast pumps, a majority of women own or use a pump to express milk (Johns et al 2016, Clemons & Amir 2010, Labiner-Wolfe et al 2008).
There are benefits to using breast pumps; however, nipple pain and damage have been identified as adverse effects, occurring at a rate of about 15-17% (Qi et al 2014, Clemons & Amir 2010).
We designed the cushion with the objective of reducing nipple pain and discomfort by softening the interface between the breast and the pump, whilst allowing the nipple and areola to move more freely within the pump.
Getting good flange size is enough, why use a cushion too?
Getting the right flange size can make a big difference to comfort and milk flow, however even with the 'right' flange size, people can still experience discomfort or pain.
In addition, a flange is still one hard piece of plastic or silicone that forces the nipple to conform to its shape and rub against it.
The Milkdrop cushions have a (patent pending) ribbing on the underside, which has a shape, softness and flexibility to allow the nipple to move with the cushion as the pump suction is on. It can feel more comfortable and more natural for some people!
The cushions can also be helpful for people who are between sizes. They are more forgiving than harder flanges, because they are so soft, and they mould more easily to the woman's shape, even as her nipple changes through lactation.
How is this different from other cushions?
Firstly, the cushion is softer and more flexible than others. It measures 10A on the Shore Hardness scale, making it 3-4x softer than existing breast pump cushions and over 10x softer than existing breast pump heads. This softness allows it to be more flexible and mould to the shape of the nipple.
Secondly, the cushion is designed with a series of ridges, which roll over when the pump is on, and allow the nipple and areola to move within the pump flange, rather than be pressed against it. This may be one reason for lower swelling and chafing that we had seen in our pilot.
Is the cushion clinically evaluated?
Milkdrop is currently being evaluated at a birthing hospital in Australia.
While we wait for the feedback from that study, we can share the initial observations from a pilot study of the cushion in 2021, which found:
- 92% of women found pumping more comfortable
- comfort increased (score: 5 /10 to 9 / 10)
- nipple swelling decreased (score: 6 /10 to 0 /10)
- nipple swelling decreased (6 /10 to 0.5 / 10), redness decreased (6 /10 to 0.5/10) and damage decreased (4/10 to 0/10) for women identifying as having an uncomfortable pumping experience.
- 73% of women collected about the same or more milk.
Based on the results of this study, we adjusted the design to remove the bulk from the cushion, and have improved the proportion of women collecting the same or more milk to about 85-90%.
Does the cushion affect milk volume?
Most women will see an increase in comfort and little change or an increase in milk volume. However, about 10% of women will see less milk flow and volume - they will need to stop using the cushions to maintain their supply if exclusively pumping. We offer full refunds for women experiencing problems with the cushions.
Can it be used between clients?
The sample set we send you can be used to size clients in your practice. Please clean according to FDA guidelines for breast pump part washing, and disinfect as you would other reusable pump parts. The cushions will last 4-6 months before needing replacement.
Can you help my clients with a discount?
Yes! We'd be happy to set you up with a discount. Although the cushions are expensive up front, they last much, much longer (4 to 6 months, rather than 4 ot 6 weeks). For people who pump for some time, they are cheaper in the long run and are less wasteful. Buy once, buy well.
Is it manufactured safely?
Milkdrop is registered with the FDA and the ARTG (#352892) as a Class I medical device.
It is manufactured with medical-grade silicone and strict quality control. It is appropriate for use on broken skin and for infants to ingest milk that has come into contact with it.
Do you do wholesale pricing?
Yes! Please read here for details on how to provide cushions for your clients in your clinic, on your website or through referrals.
Sizing & Compatability
Which pumps are they compatible with?
Milkdrop cushions fit most flange sizes on most traditional (and soon, wearable) pumps. See the list of compatible pumps here.
Which size should I give my client?
You will receive our 4x sample sizes (XS, S, M, L) ranging from 15mm to 30mm nipple diameters. Most people fit a size S, followed by XS and M. We rarely send out L cushions, and always double check that they are measuring the nipple, rather than the areola or flange size first.
Here's a video on how to size the cushions.
The sizes are based on nipple diameter, not areola diameter, breast size or flange size. You don't need to add 1-2mm either side for Milkdrop cushions.
Which flanges do they fit with?
Milkdrop cushions fit most flange sizes on most pumps. See the list of compatible pumps here. While most people will be comfortable on the standard flange size with their pump (usually 24mm), the cushions do work best on the smallest available, comfortable, flange size for each person without the cushion**.
For example, if your client measures 19mm across the base of the nipple, then a S cushion on a 19 or 21mm flange is typically the most comfortable. Other inserts or cushions need to be removed before placing on the Milkdrop cushion, but simple sizing inserts that only change the tunnel size are fine to use! Some people prefer 24mm flanges with size inserts because it keeps the cushion centred nicely.
**This is particularly important for people with elastic nipples, so that their nipple is supported once it has passed through the cushion. It is also important for people with flatter or inverted nipples.
How do I know if they have the right fit?
As with many pumping related sizing issues, the right fit should be comfortable and should yield good milk flow for that person.
It also takes some trial and error to find the right fit. They should only need to pump for a minute or less to figure out fit. Here are some experiences and fixes:
- If they feel like their nipple is being restricted from moving -> try the size up.
- If they feel like their areola is being sucked through or they have swelling of the areola after pumping -> try the size down.
- If the cushion size feels good but they see or feel their nipple swelling once it passes through the cushion -> try a smaller pump flange size/size insert. People with more elastic nipple tissue need to use flange sizes close to their 'correct' size, rather than the usual 24mm flange.
- If the cushion size feels good but they see the cushion start to move off-centre or slip towards the tunnel -> try a silicone size insert. People with flatter or inverted nipples or smaller breasts may be more prone to the cushion moving off-centre. Here's a video explaining how to use a sizing insert with a cushion.
If they experience any pain, please stop using the cushions and contact us. If you have any questions about sizing, please contact us at support@milkdroppumps.com
Try a set of cushions in your clinic
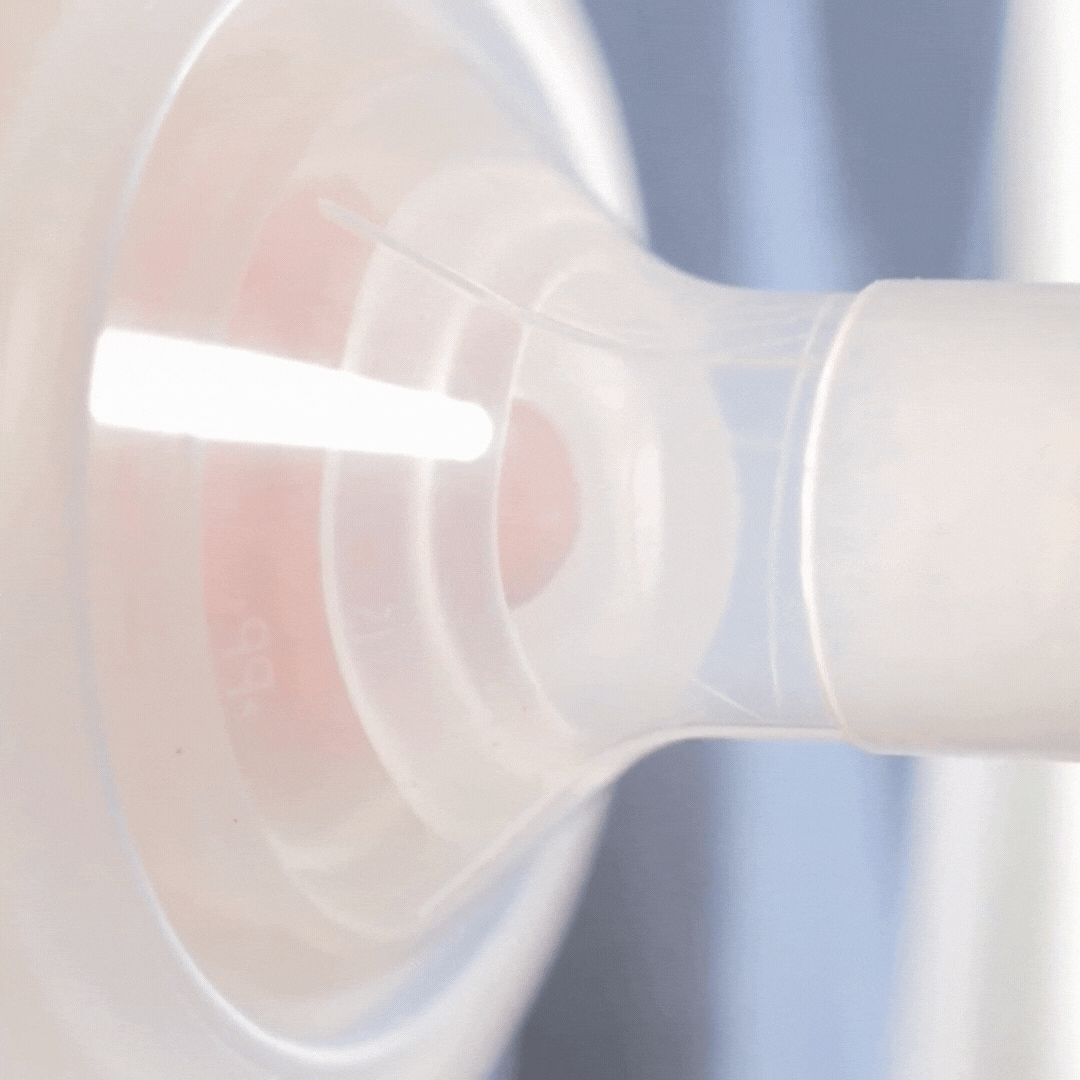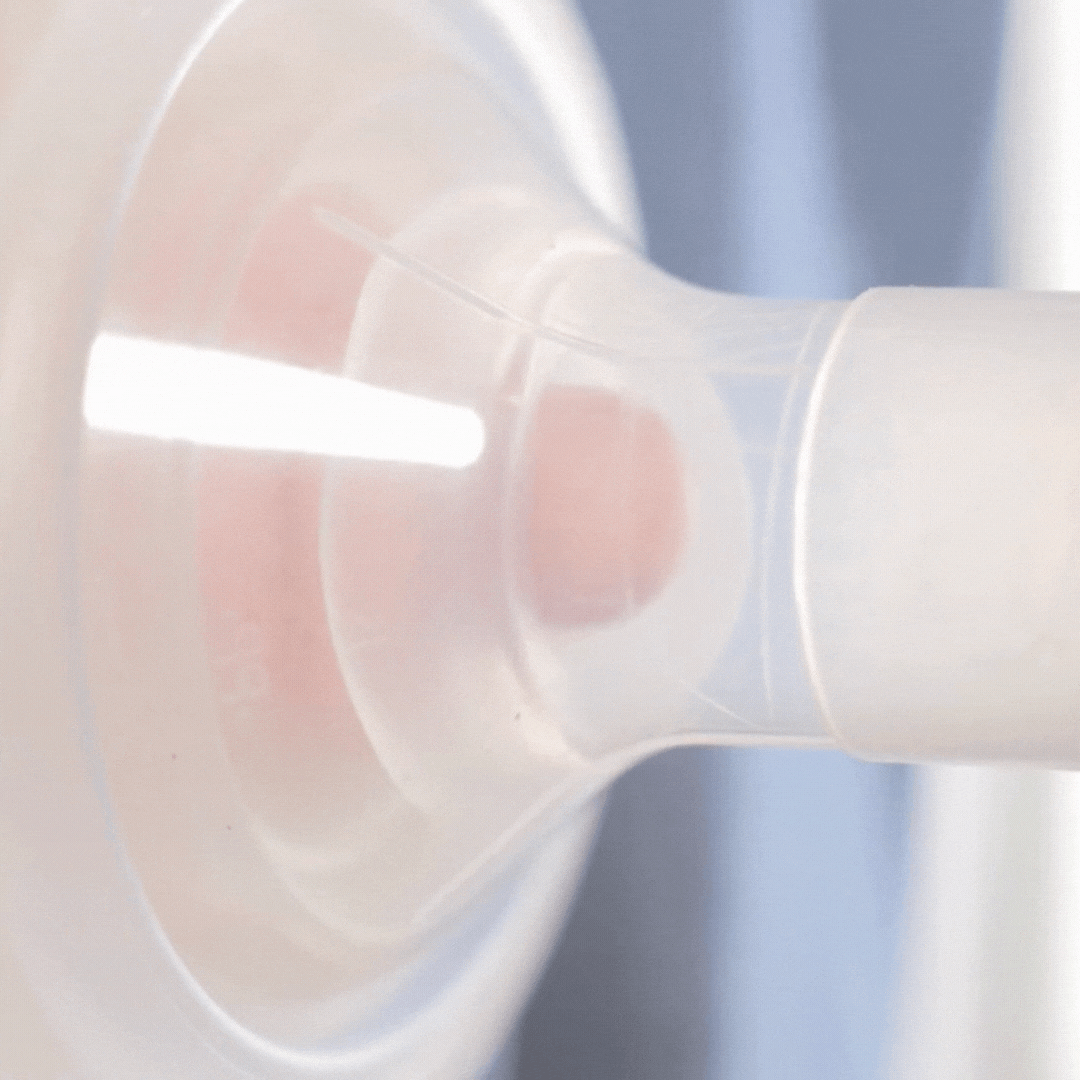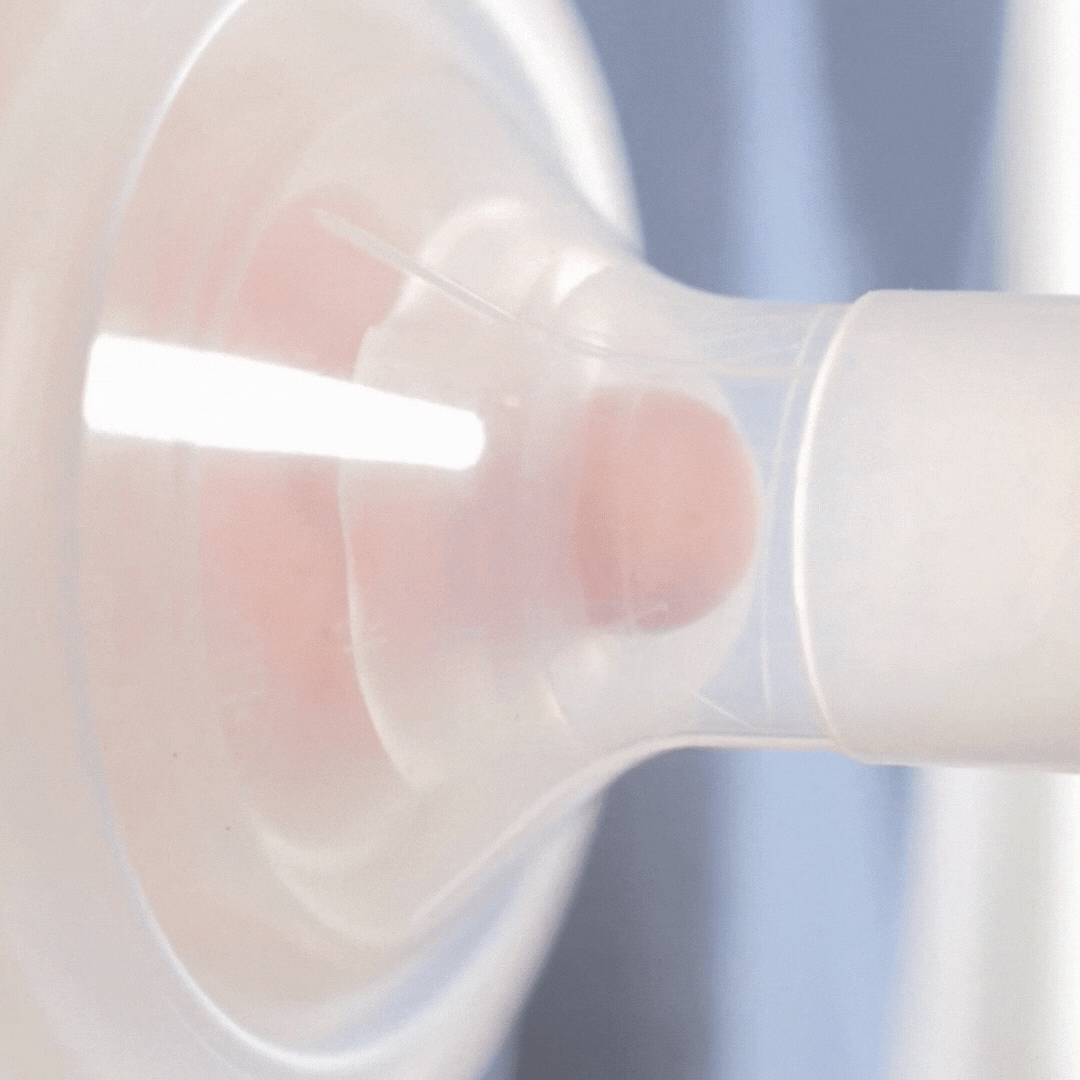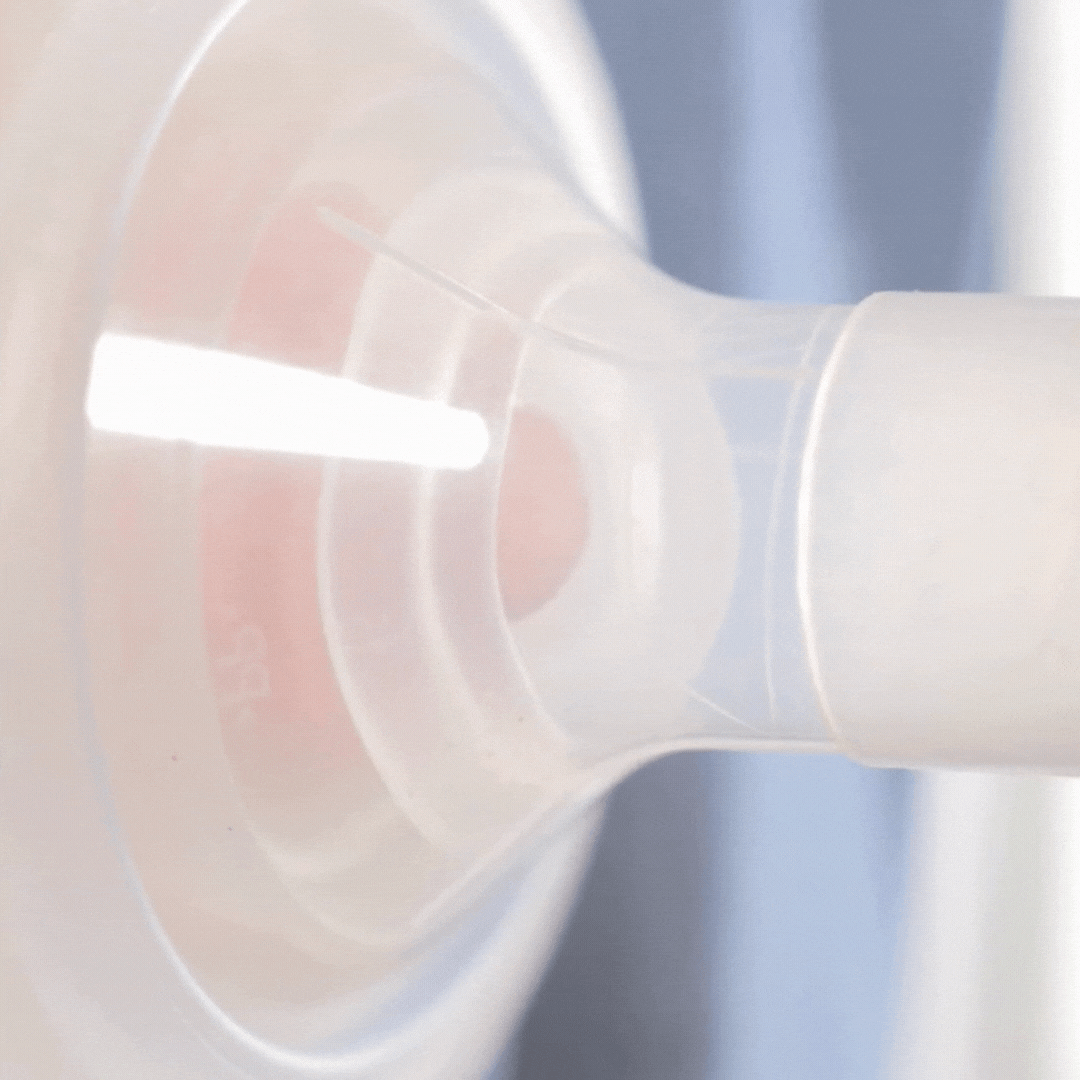
How it works
This video shows how the cushion moulds to the nipple shape. It allows the nipple to draw through, but the areola to stay supported (although still moving). Notice how the cushion compresses as the pump sucks. This cushion is placed on a standard flange (24mm). Even though the flange is too wide for the nipple, the cushion is providing support.


